


Lipopolysaccharide (LPS) has been reported to have endotoxic properties that can be utilized in the context of vaccine adjuvants. CD BioGlyco has long focused on the development of LPS-based adjuvants to help customers improve vaccine efficacy. Our efficient and professional services will accelerate our customers' research progress in developing novel adjuvants.
LPS, commonly referred to as endotoxins, represent a broad and highly heterogeneous group of bacterial outer membrane glycolipids from Gram-negative bacteria such as Neisseria meningitidis, Escherichia coli and Salmonella minnesota. LPS consists of a core oligosaccharide, an externally repeating O-antigen polysaccharide chain, and a highly conserved TLR4-activated lipid A. It is a potent inducer of innate immunity as well as a potent enhancer of the immune response against protein antigens.
This adjuvant activity can be utilized after immunization with bacterial-derived vaccines that naturally contain LPS, and when LPS or its derived molecules are added to the purified vaccine antigens. However, the endotoxin activity of LPS seriously limits its use as an adjuvant, resulting in unacceptable reactogenicity. At present, progress has been made in the chemical treatment of purified LPS or genetic modification of LPS biosynthesis to weaken LPS and isolate endotoxin and immunostimulatory activity.
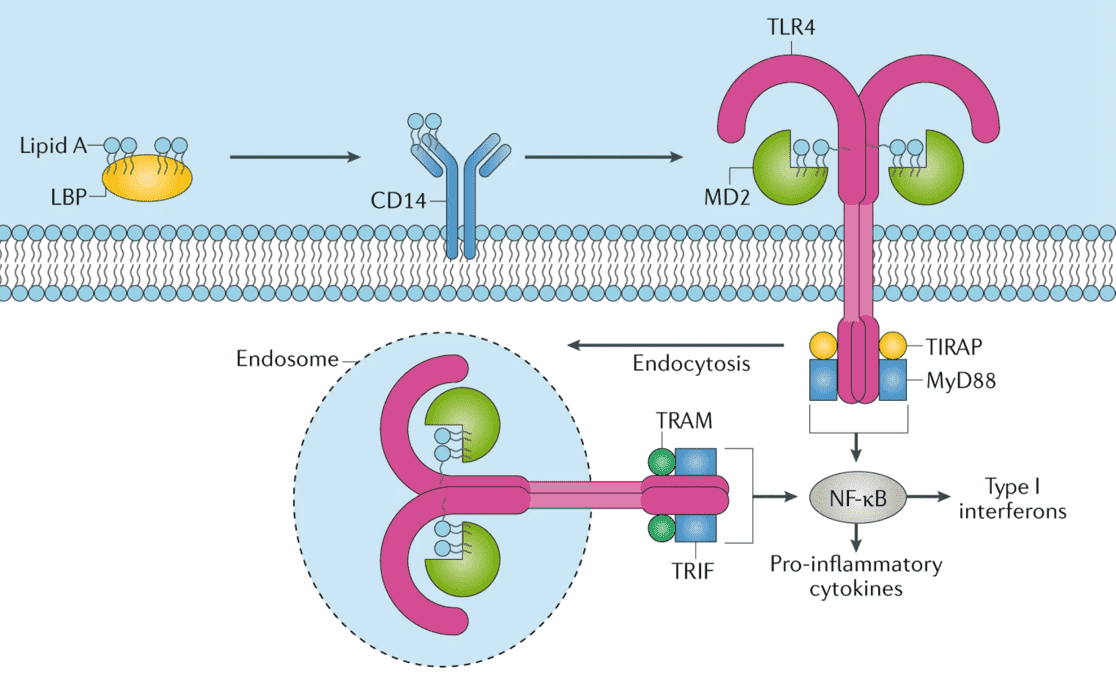 Fig.1 Schematic representation of LPS/Toll-like receptor 4 (TLR4) signalling pathway and lipid A mechanism of action. (Pifferi, 2021)
Fig.1 Schematic representation of LPS/Toll-like receptor 4 (TLR4) signalling pathway and lipid A mechanism of action. (Pifferi, 2021)
Based on the current extensive coverage of bacterial genes involved in LPS biosynthesis and modification, CD BioGlyco is committed to performing a wide range of modifications to the basic structure of LPS to develop potentially safe adjuvants. Our services include but are not limited to:
CD BioGlyco offers a wide range of services for our clients in the field of glycobiology. We have made great progress in the development of LPS-based adjuvants and our services have been recognized and trusted by our clients. If you are interested in our services, please contact us directly for more information.
Reference:
