



F and G proteins are the main structures of MPV. The F protein is found to be an important protective antigen of MPV, capable of inducing protective immunity in the body. It does not depend on other vesicle membrane proteins to mediate membrane fusion. G protein plays an auxiliary role in virus adsorption mediated by F protein. It is immunogenic in its own right but does not induce a protective immune response on its own. G protein has also been shown to have good immunogenicity. Vaccines are an effective way to prevent MPV. F and G proteins are ideal subunit vaccine antigens. A study evaluates an avian poxvirus vaccine with recombinant F protein. The vaccine is shown to stimulate the production of specific antibodies in turkeys and to be immunoprotective.
 Fig.1 Model structure and proteins encoded by Human MPV. (Wikipedia)
Fig.1 Model structure and proteins encoded by Human MPV. (Wikipedia)
CD BioGlyco establishes a comprehensive sugar-related Vaccine Development Platform. Vaccine design, structural characterization, impurity studies, activity analysis, etc., are all available. As important target antigens for cytotoxic T-lymphocytes, the immunogenicity of F and G proteins has been extensively studied. Both proteins can be used for MPV vaccine development. We provide MPV vaccine development services based on F and G proteins.
Genetically engineered vaccines constructed with modern biotechnology have been widely recognized for their safety, absence of regression, and low cost. We mainly provide MPV Recombinant Subunit Vaccine development services. In order to improve the safety and immunogenicity of the vaccine, we screen and construct recombinant F and G proteins. Both proteins can be produced by protein expression systems. We have a variety of expression systems to choose from.
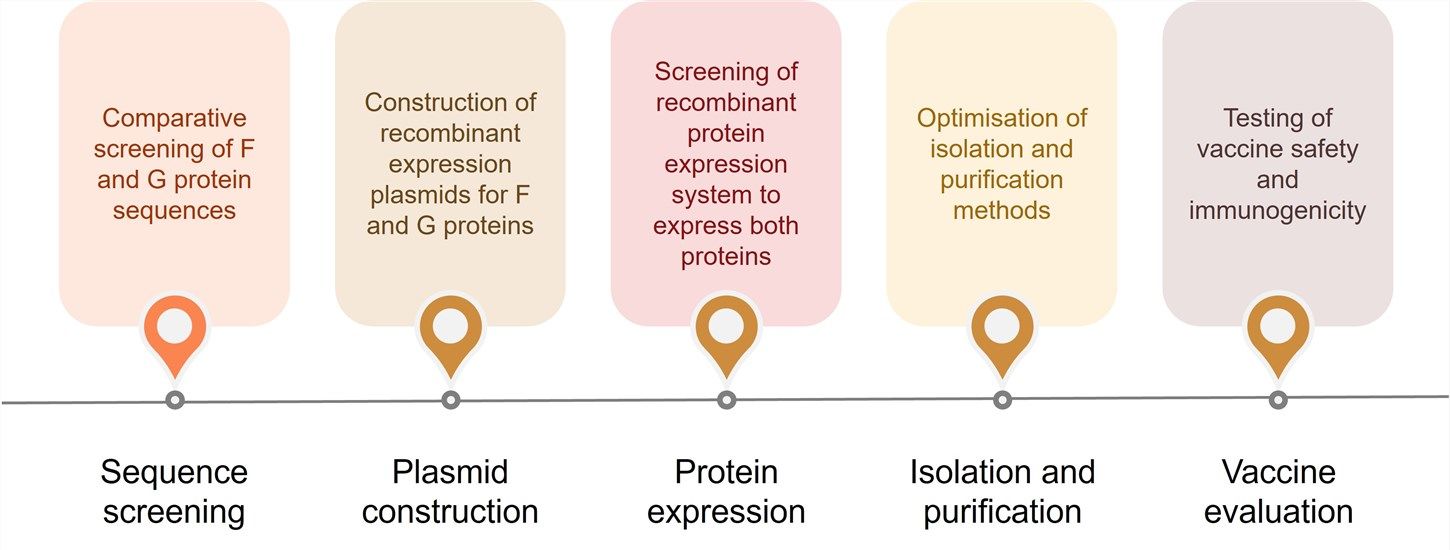 Fig.2 Process of MPV vaccine development. (CD BioGlyco)
Fig.2 Process of MPV vaccine development. (CD BioGlyco)
It is characterized by high expression, safety, and non-toxicity. A variety of genes can be efficiently expressed in it. The system also has other advantages: stable expression of exogenous proteins, a single component of the culture medium, easy to operate, spatial folding of the expressed proteins, etc.
The Mammalian cell expression system is able to provide post-translational modifications for recombinant proteins that are closer to the natural state. It has the spatial structure and modifications necessary for active proteins. The exogenous protein produced by this system is closer to the natural protein.
Studies show that both multivalent recombinant vaccines and nucleic acid vaccines expressed using the E. coli system have some immunoprotective effect.
Isolation and purification are very critical tasks. We use an optimized protein purification method, which can improve the speed and quality of purification. We have a comprehensive vaccine evaluation system to support preclinical studies. We will evaluate the vaccine in terms of the optimal immune dose, immune duration, shelf life, safety indicators, etc.
CD BioGlyco is a trusted biopharmaceutical company and we have established multiple routes to sugar-related vaccine development. Please feel free to contact us if you have any needs in sugar-related vaccine development. We look forward to working with you.
References
