



CD BioGlyco offers a wide range of Pharmaceutical and Biological Analysis Services. With our advanced technology and experienced experts, we conduct a comprehensive evaluation of the properties and functionality of powdered cellulose. Powdered cellulose is a form of cellulose, which is made from cellulose. It is a substance with the same properties as cellulose and microcrystalline cellulose. It has wide applications in the pharmaceutical field as a pharmaceutical excipient. Powdered cellulose is a white or nearly white, odorless, tasteless powder that is non-toxic and non-irritating. Its molecular weight varies as the length of the molecular chain varies. The following is a detailed introduction to our analysis services for powdered cellulose.

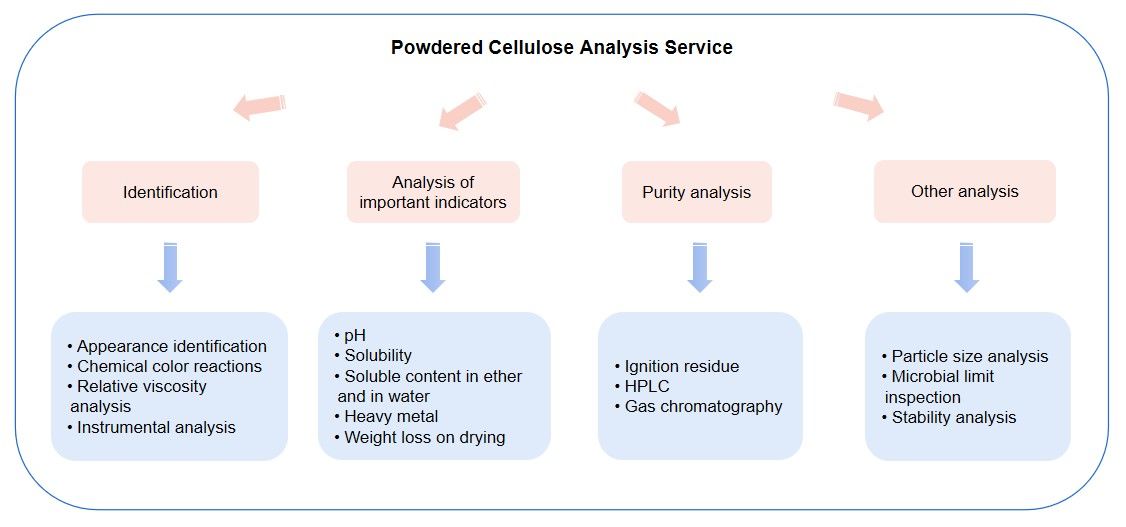
We use a variety of identification methods to ensure the authenticity and accuracy of powdered cellulose.
We strictly control and analyze multiple important indicators of powdered cellulose to ensure its quality is stable and reliable.
Purity is one of the important quality indicators of powdered cellulose as a pharmaceutical excipient. We use multiple methods to analyze its purity comprehensively.
In addition to the above analysis services, we also provide the following other analysis services to meet the different needs of our clients:
Technology: Characterization of the physicochemical properties of cellulose
Journal: Nanomaterials
IF: 3.791
Published: 2020
Results: The authors capitalized on the biodiversity of Ecuador to assess microcrystalline cellulose derived from borojó (Alibertia patinoi), an indigenous plant, for its potential as a solid dosage formulation excipient. Initially, they conducted scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy, and X-ray diffraction (XRD) to juxtapose the structure and attributes of the extracted cellulose with two benchmark commercial variants. Subsequently, the authors implemented quality checks to determine the isolate's suitability as an excipient, including fluidity, hardness, friability, and disintegration tests. The cellulose extracted from the indigenous plant demonstrated characteristics akin to commercial and microcrystalline cellulose, establishing it as a potential excipient for the pharmaceutical sector. Finally, the authors carried out a dissolution test and deduced that all tablets displayed a rapid release time of the active principle.
 Fig.1 Fourier-transform infrared spectra of powders of the three excipients. (Viera-Herrera, et al., 2020)
Fig.1 Fourier-transform infrared spectra of powders of the three excipients. (Viera-Herrera, et al., 2020)
Powdered cellulose is primarily used as a diluent in tablets and hard capsules to increase the volume of formulations that contain too little drug. In addition, it is used to reduce the settling rate of oily suspension-filling liquids in soft capsules. At the same time, powdered cellulose is also used as an excipient for powders, a suspending agent for oral aqueous suspensions, and to reduce drug sedimentation in the preparation of suppositories.
It is worth noting that, unlike the properties of general powdered cellulose, powdered cellulose with low crystallinity can be used as an excipient for direct compression.
The weight loss on drying of powdered cellulose is one of the important indicators to evaluate its drying degree. Excessive weight loss on drying may cause problems such as moisture absorption and agglomeration in pharmaceutical preparations during storage and use, affecting the stability and effectiveness of the drug. Therefore, by measuring the drying weight loss of powdered cellulose, we judge whether its drying degree meets the requirements, thereby ensuring the quality and stability of pharmaceutical preparations. At the same time, the results of weight loss on drying can also provide an important reference for the preparation process of pharmaceutical preparations.
At CD BioGlyco, we understand the importance of Pharmaceutical Excipient Analysis for pharmaceutical preparations. For the pharmaceutical excipient powdered cellulose, we provide comprehensive analytical services to ensure that its quality meets standards. We always adhere to client-centeredness and provide clients with high-quality and efficient services. Please feel free to contact us if you are interested in our powdered cellulose analysis service.
Reference
