Tumor-Associated Fucosyl-GM1 Ganglioside Antigen Production Service
Struture of Fucosyl-GM1 Ganglioside
Fucosyl GM1, a glycosphingolipid molecule, exhibits an intriguing pattern of expression in cancer biology. Notably, it is absent from normal tissues but prominently displayed on the cell surface of small-cell lung cancer (SCLC). SCLC is a highly aggressive subtype that accounts for approximately 10-15% of all lung cancer cases. Due to its high prevalence in SCLC and significant impact on mortality rates, fucosyl GM1 has become an extremely enticing target for therapeutic interventions.
 Fig.1 Structure of prioritized ganglioside cancer antigen fucosyl GM1. (CD BioGlyco)
Fig.1 Structure of prioritized ganglioside cancer antigen fucosyl GM1. (CD BioGlyco)
Tumor-Associated Fucosyl-GM1 Ganglioside Antigen Production Service at CD BioGlyco
We provide the synthesis service of fucosyl-GM1 from LacβSph through a four-step sequential OPME reaction, with a high total yield.
- Firstly, a one-pot two-enzyme sialylation system, involving NmCSS and PmST3, is utilized to obtain GM3βSph without the fatty acyl chain. This system is capable of utilizing both oligosaccharides and glycolipids as acceptor substrates. Purification is achieved using a simple C18-cartridge SPE method, resulting in a gram-scale production of pure GM3βSph. The remaining LacβSph is also recovered during this process.
- Subsequently, we employ a one-pot four-enzyme system consisting of recombinant Bifidobacterium longum strain ATCC55813 N-acetylhexosamine-1-kinase (BLNahK), Pasteurella multocida N-acetylglucosamine uridylyltransferase (PmGlmU), P. multocida inorganic pyrophosphatase (PmPpA), and Campylobacter jejuni β1-4-N-acetylgalactosaminyltransferase (CjCgtA). This enzymatic cascade facilitates the glycosylation of GM3βSph, resulting in the formation of GM2βSph. To purify GM2βSph, we utilize a C18 cartridge, passing the reaction mixture through it and subsequently eluting it with a CH3CN solution in water.
- In the subsequent step, GM1βSph is synthesized from GM2βSph through the utilization of a one-pot four-enzyme system for galactose activation and transfer. This system comprises Escherichia coli galactokinase (EcGalK), B. longum UDP-sugar pyrophosphorylase (BLUSP), P. multocida inorganic pyrophosphatase (PmPpA), and C. jejuni β1-3-galactosyltransferase (CjCgtB).
- With the glycosylsphingosines, we investigate the conditions for efficient acylation to generate the desired glycosphingolipids. By subjecting glycosylsphingosines to reactions with palmitoyl chloride in a mixture of tetrahydrofuran (THF) and saturated NaHCO3
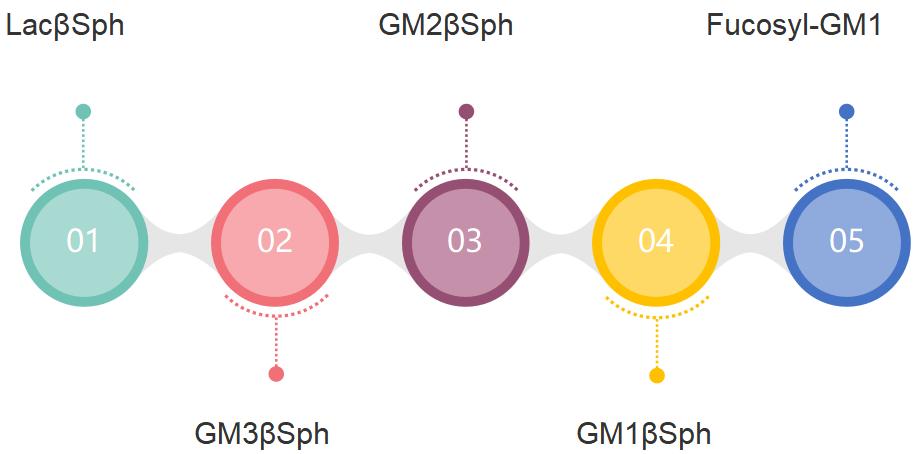 Fig.2 The primary steps involved in the synthesis of fucosyl-GM1. (CD BioGlyco)
Fig.2 The primary steps involved in the synthesis of fucosyl-GM1. (CD BioGlyco)
Based on the fucosyl-GM1 antigen, we prepare a conjugate to study the anticancer vaccine. To boost the immunogenicity of the fucosyl-GM1 antigen, we employ a chemical conjugation method to connect it to keyhole limpet hemocyanin (KLH), a protein derived from shellfish. This is then combined with the immunological adjuvant QS-21. Additionally, we utilize a mouse model for the vaccination of our conjugate and perform serological analysis on the subjects.
 Fig.3 The steps of serological analysis. (CD BioGlyco)
Fig.3 The steps of serological analysis. (CD BioGlyco)
Applications
- Fucosyl GM1 can be used to explore the development of targeted vaccines aimed at eliciting a specific immune response against SCLC cells.
- The presence of fucosyl GM1 on the surface of SCLC cells provides an opportunity to design antibodies for the detection and diagnosis of this aggressive cancer subtype.
- Fucosyl GM1 is useful as a therapeutic target for advancing personalized medicine and enhancing outcomes for patients suffering from this severe form of lung cancer.
Advantages
- The use of a one-pot multi-enzyme reaction system reduces the number of steps and time required for synthesis, thereby improving efficiency.
- Each step in the synthesis process achieves high yields, resulting in an overall yield of up to 93% from GM3βSph to the final product Fuc-GM1βSph.
- By adjusting reaction conditions and enzyme amounts, selective glycosylation of different substrates can be achieved, allowing for the synthesis of various types of glycosphingolipids.
- The reaction process proceeds under mild conditions without the need for high temperatures or strong acid/base conditions, which helps to protect sensitive sugar moieties and substrate structures.
- This method can be applied to the synthesis of complex glycosphingolipid structures, providing convenience for further glycolipid research and applications.
CD BioGlyco possesses the Glyco™ Vaccine Development Service Platform to explore the field of glycoscience. By using advanced techniques and cutting-edge methodologies, we can efficiently synthesize and purify high-quality ganglioside antigens for various applications. We also offer customized solutions to meet the specific needs of researchers and companies. If you would like more information about our services, please don't hesitate to contact us.
Reference
- Yu, H.; et al. Streamlined chemoenzymatic total synthesis of prioritized ganglioside cancer antigens. Organic & biomolecular chemistry. 2018, 16(22): 4076-4080.
This service is for Research Use Only, not intended for any clinical use.



 Fig.1 Structure of prioritized ganglioside cancer antigen fucosyl GM1. (CD BioGlyco)
Fig.1 Structure of prioritized ganglioside cancer antigen fucosyl GM1. (CD BioGlyco) Fig.2 The primary steps involved in the synthesis of fucosyl-GM1. (CD BioGlyco)
Fig.2 The primary steps involved in the synthesis of fucosyl-GM1. (CD BioGlyco) Fig.3 The steps of serological analysis. (CD BioGlyco)
Fig.3 The steps of serological analysis. (CD BioGlyco)