


Protein N-glycosylation plays an important role in pathological and physiological processes. Characterizing N-glycosylation becomes extremely necessary in protein research. CD BioGlyco has excellent technology platforms and unique database to meet the customer's research needs and obtain reliable results. We have confidence to be your essential research assistant in the field of glycobiology.
Glycosylation is one of the most common post-translational modifications spanning more than 50% of the proteome, and its role in diseases such as cancer, infectious diseases and cardiovascular diseases has been confirmed in numerous studies. Glycan microheterogeneity (site-specific glycan structure) and macroheterogeneity (glycosylation site occupancy) form the integral heterogeneity of proteins which is essential to understand the biosynthesis and function of glycoprotein. N-glycosylation site occupancy reflects the proportion of N-glycosylation in the protein, and this phenomenon in turn reveals the availability of sites, substrate concentrations and enzyme kinetics in ER. The properties of glycoprotein are largely affected by the site occupancy, and defects in site occupancy may be detrimental to physiological function.
N-glycosylation site occupation analysis in CD BioGlyco, including but not limited to the following technologies.
Liquid chromatograph mass spectrometry (LC-MS) based quantification has become widely used and considerably advanced quantification of N-glycosylation site occupancy in samples which labeling and label-free methods are frequently used. Stable isotope labeling by amino acids in cell culture (SILAC), tandem mass tag (TMT), and dimethylation are commonly used to introduce 'heavy' and 'light' isotopes or isobaric tags into samples, while label-free methods are based on spectral counting or peptide-ion intensity.
Our company adopts matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) technologies, combined with bioinformatics algorithms, to achieve highly sensitive localization of glycoproteins' N-glycosylation sites, quantification of site occupancy, and structural analysis of the glycan, and to facilitate the study of glycobiological mechanisms.
Technology: MALDI-TOF-MS
Journal: Scientific Reports
Published: 2019
IF: 3.8
Results: This paper presents the first systematic comparison of site-specific N-glycosylation profiles of glycoproteins in HeLa cells by MALDI-TOF-MS combined with N-glycosylation profiling. The researchers identified 69 N-glycosylation sites and 178 glycoforms of 43 human glycoproteins, revealing the modification characteristics of HeLa glycoproteins with high fucosylation (68.7%) and moderate sialylation (46.8%). Meanwhile, the researchers found that glycosylation patterns differed significantly between proteins and between different sites of the same protein.
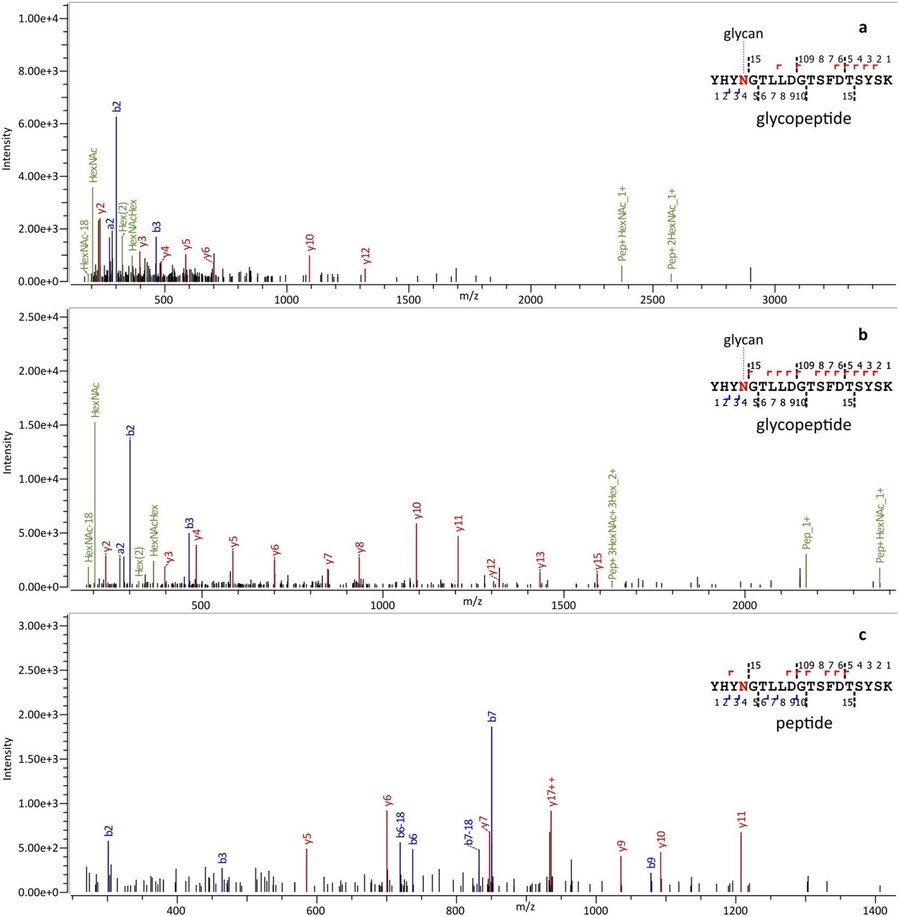 Fig.1 Example MS/MS spectra of glycopeptides. (Turiák, et al., 2019)
Fig.1 Example MS/MS spectra of glycopeptides. (Turiák, et al., 2019)
CD BioGlyco provides diverse and systematic glycosylation site occupation analysis services. The efficient services and accurate results have gained a good reputation worldwide for our company. We will continue to improve the quality of services and bring convenience to customers' glycobiology research in order to save time and funds for customers while obtaining credible results.
Customers can contact our employees directly and we will respond promptly. If you are interested in our services, please contact us for more detailed information.
Reference
