


GPI-AP is a class of proteins that are anchored to the surface of the cell membrane through its carboxy-terminal GPI structure without crossing its phospholipid bilayer structure. The GPI portion consists of a conserved core glycan, phosphatidylinositol, and sugar side chains. A large number of GPI-APs are found in vertebrates, plants, mollusks, insects, etc. GPI-APs have a variety of biological functions. They are mainly involved in cell-cell and cell-environment interactions, such as signaling, cell adhesion, complement regulation, etc. It has been found that many CD molecules are GPI-APs themselves. Others must interact with GPI-APs to realize their biological functions. Currently, GPI-APs are mainly used in cell biology, molecular biology, and disease research. Clinically, GPI-APs can be used as tumor markers in the form of intact GPI-APs present on the surface of tumor cells. They can also be present in the serum, urine, and secretions in the form of lost anchors.
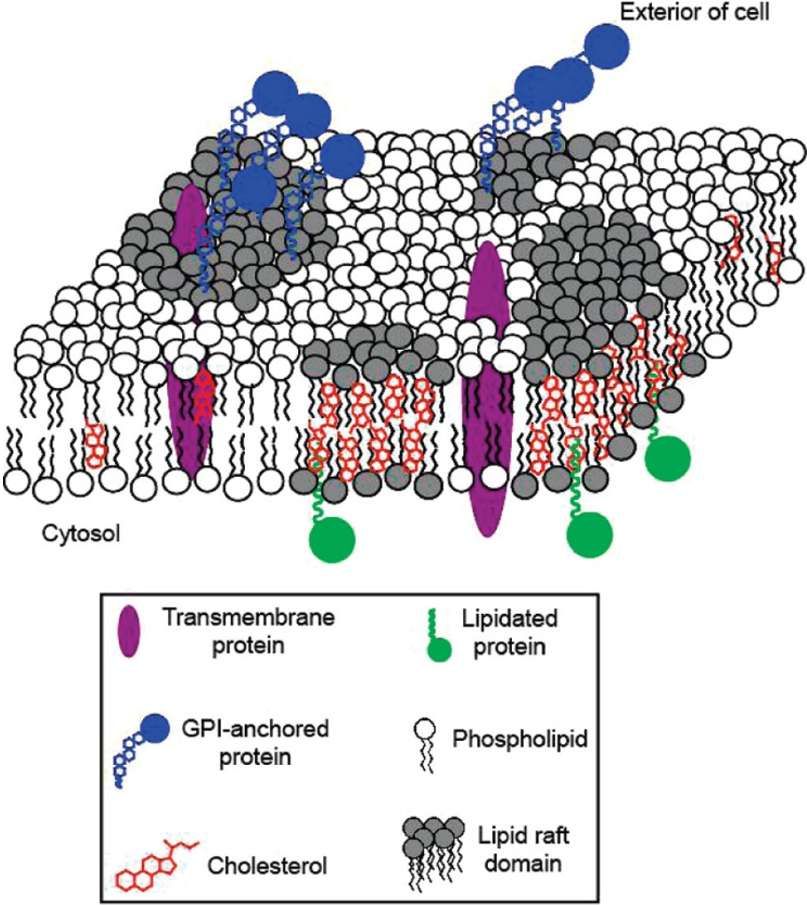 Fig.1 GPI-anchored proteins and others are believed to be associated with lipid rafts. (Paulick & Bertozzi, 2018)
Fig.1 GPI-anchored proteins and others are believed to be associated with lipid rafts. (Paulick & Bertozzi, 2018)
CD BioGlyco is experienced in glycoengineering. We develop a range of glycoengineering services for GPI-AP in order to facilitate our clients in drug discovery, functional studies, and more. We also have a wide range of detection equipment, including liquid chromatography-mass spectrometry (LC-MS), etc. Different types of biological samples can be detected.
We use gene editing techniques such as CRISPR/Cas9 to Knock Out or Overexpress GPI-AP synthesis-related enzyme genes. The modification level and structure of cell surface GPI-AP can be altered by this method, ultimately changing its biological function.
We provide GPI Anchoring-based yeast cell surface display services. Yeast cell surface display technology is a kind of eukaryotic protein expression system. It utilizes the yeast cell surface display system to anchor and display heterologous eukaryotic proteins on the surface of yeast cells. This technology has been widely used in many fields, such as protein-directed evolution, protein library screening, high-affinity antibody sorting, etc. We introduce exogenous target protein gene sequences into yeast cells after the fusion of exogenous proteins with specific vector gene sequences. Then we utilize the yeast intracellular protein transport mechanism (GPI anchoring) to express and localize the target proteins on the yeast cell surface.
We use chemical or enzymatic methods to degrade the target protein and cover the target protein sequence as completely as possible. Then the glycosylation sites in that protein are analyzed after the glycosylase is cut down. After identifying the peptide where the glycosylation modification occurs, the target protein is re-enzymatically cleaved and the sugar group on the peptide is retained. The glycotype of the site is then analyzed accurately. We purify and characterize the GPI structure in our samples based on biochemical and mass spectrometry methods. GPI structure analysis includes its monosaccharide fraction, PI, glycan structure, etc.
 Fig.2 Process of cell surface GPI-AP glycoengineering. (CD BioGlyco)
Fig.2 Process of cell surface GPI-AP glycoengineering. (CD BioGlyco)
CD BioGlyco has a variety of glycoengineering services. Besides GPI-AP, we also provide Complex GPI Anchor, Hydroxy Fatty Acid GPI Anchor, and other Glycoengineering Services. If you would like to know more about GPI-AP service or you encounter any problems, please feel free to contact us. Members of our team will get back to you as soon as possible.
Reference
